Life History & Behaviour
Skip to: Reproduction , Development , Feeding , Locomotion and movement , Sensory perception and response (including short experimental investigation).
Reproduction
All Diadema species are gonochoric (unisexualist; individuals are one of two sexes), however do not show external sexual characteristics so sexing individuals in the field is nearly impossible (Pearse & Cameron, 1991). As they are ‘regular’ urchin species, all Diadema have 5 gonads, each suspended underneath one of the 5 interambulacral regions of the test (Ruppert et al, 2004). Each gonad is connected to the external environment via a gonopore located on a genital plate,which is linked to the gonad via gonoduct (see Anatomy). The production of sperm from spermocytes, and ova from oocytes takes male and female D. savignyi 1 month to complete (Muthiga & McClanahan, 2007). After completion, sperm and eggs are released into the water column for external fertilization. Most species of Diadema have monthly spawning cycles; and D. savignyi has been shown to spawn around the new moon (Muthiga & McClanahan, 2007). D.savignyi reproduce throughout the year, but may peak at a certain time to avoid reproductive times of competing urchin species (Muthiga & McClanahan, 2007).
Development
Fertilization leads to the development of a ciliated and free-swimming blastula within 12 hours (Ruppert et al, 2004). After gastrulation, in which larval muscles, skeleton and a coelom are formed, the planktotrophic larva emerges. This echinopluteus larva bears four pairs of long arms supported by skeletal rods, and uses these ciliated arms to swim and feed. Within several months later, the juvenile rudiment forms inside the larval body, and the echinopluteus becomes developmentally competent to metamorphose (Ruppert et al, 2004). The larva sinks to the substratum and uses a variety of stimuli to assess the suitability of the microhabitat for settlement. These cues are usually chemical, and may come from algae films, microbial films or macroalgae (Cameron & Hinegardner, 1974). If the microhabitat is unsuitable, larvae can swim away to assess other locations (Burke, 1980). Once it has found a suitable microhabitat, the larva attaches with tube feet that extend from the rudiment, and metamorphoses into a juvenile urchin within an hour (Ruppert at al, 2004).
Feeding
Diadema are nocturnal, so feeding activity is greatest during the evening and night (Tuya et al, 2004a). Using their highly developed jaw apparatus and protruding teeth, Diadema graze the substratum in search of algae or other plant and animal material (Rupert et al, 2004). Research on the feeding preference of Diadema suggests that these urchins prefer red and brown algae, in particular Galaxaura, Halopteris, Lobophora, Dyctiotia,Padina, and Halymenia, while they tend to avoid Sargassum, Turbinaria,Boodlea and Chaetomorpha (Ndibelema & Shunula, 1986; Randall, 1961; Tuya et al, 2001). Apart from grazing, there is also some evidence of Diadema using their spines to trap drifting algal particles for feeding (Littler et al,1983). While studying D. savignyi on Heron Island, I believe I may have observed a similar behavior; the specimen waved its spines in circular motions for an extended period of time, and sediment and particles seemed to be trapped on the spines.

D. savignyi that was seen waving its spines in the water
column, possibly to trap particles for feeding.
Locomotion and movement
Both the spines and tube feet (parapodia) are used in urchin locomotion (Ruppert et al, 2004). The parapodia are powered by the water vascular system, which pumps water in and out of the foot to move the urchin. Water is pumped into the parapodia to extend the foot, the foot then uses its suction cup to adhere to the substrate, the urchin is pulled forwards, and the water is drawn away from the foot, breaking the seal. Spines, controlled by muscles within the test, can also be used in movement simply by pushing against hard surfaces.
Action of the tube feed in Echinoid locomotion:
a) The protractor muscles in the ampulla relax, and retractor muscles in the foot contract, shortening the foot and forcing the fluid into the ampulla. Muscles on one side of the foot relax, while the other side contracts, moving the foot ahead. b) Protractor muscles contract, while retractor muscles relax, causing fluid to fill the foot and extend it. The foot swings through an arc and glues itself to the substrate at the bottom. The animal uses muscles to pull itself over the foot. c) Chemical secretions degrade the glue and the foot releases and swings upward for the cycle to begin again.
Sensory perception and response
The nervous system of Diadema and most other Echinoids consists of a central nerve ring that encircles the pharynx, and radial nerves that spread out from the ring and run along the test (Ruppert et al, 2004). There are sensory cells in the epithelium, on the spines, pedicellariae and tube feet. Mostly all Echinoids respond to light, and actively seek shade. Millot (1954) described the response of D. antillarum to changes in light intensity, and found that the tube feet and ambulacral margins of the test are most sensitive. He also found that when partly shaded, the specimens moved their spines towards the shaded area, and wiggled them erratically for a period of time.
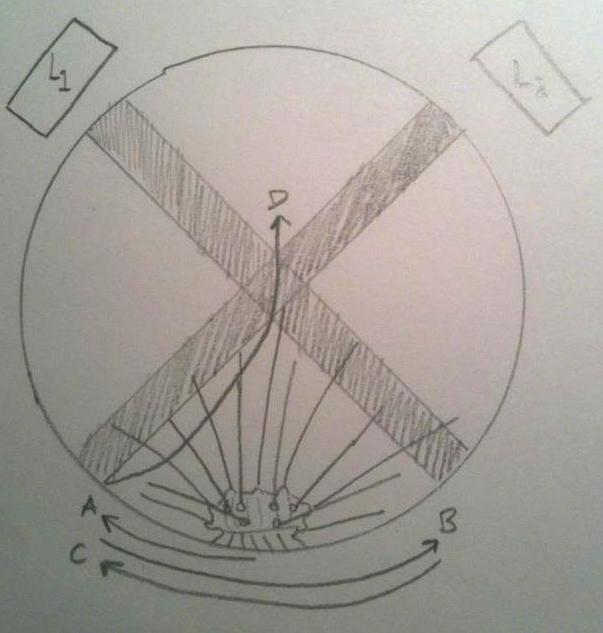 
The behaviour of a dark-adapted Diadema when placed between two light beams, L1 and L2. The urchins movement can be tracked from A to B to C, and finally to D, where it came to rest. Note how it perceives the light beams and moves away from them, and comes to rest in the shade.
Image appropriated from Millet, 1954.
A short experimental investigation was performed on Heron Island in September of 2012 on D.savignyi to investigate the relative abilities of the pigment cells on the spines and test of these specimens to respond to light.
Short experimental investigation
INTRODUCTION
The Western Basin Dredging and Disposal Project in Gladstone Harbour is currently under expansion and has approval to dredge 46 million cubic meters from the harbour over the next 20 years (Gladstone Ports Corporation, 2012). This has the potential to have massive effects on nearby reefs, including Heron Island Reef, due to increased sedimentation and run-off (Johannes, 1975). Increased sedimentation leads to increased sediment load on corals and other benthic organisms (Rogers, 1990). One such organism that maybe susceptible is the Diadema urchin.This species relies on light perception to seek out shady crevices, for predator avoidance and protection from excessive wave action (Rogers,1990). With increased sediment load covering the light-sensitive urchins test, responsiveness to light may be reduced and the urchin may not be able to successfully find shelter. This could lead to increased predation or damage due to wave action, and may result in declining abundance of Diadema on the reef.
This experiment aimed to determine effect that increased sediment load has on the light responsiveness of Diadema.
METHODS
A total of 5 Diadema specimen were collected from the reef flat and reef crest of Heron Island Reef, specifically 2 D. savignyi and 3 Echinothrix diadema. One E. diadema specimen was not considered fit enough to provide typical behavioral results, so was omitted from the experiment. Urchins were left in a dark, lidded tank in the shade for 15 minutes for dark acclimation. Urchins were then placed into another lidded tank that was half in direct sunlight and half in the shade. Once the urchin was placed at the starting point on the sunny side, the lid was removed and the urchin was in direct sunlight. The time taken for each urchin to travel from the start point in direct sunlight to the shade was recorded. Urchins were left to rest for 4 hours to prevent any interference of fatigue on results. Urchins were again acclimatized to darkness and then placed in the light/dark tank; however this time were covered in a thin layer of sediment before exposure to direct sunlight, in order to block the light-sensing cells on the test. The time taken to cross into the shade was again measured. Both measurements were compared in order to obtain a time difference for each urchin to determine the effect that increased sediment load has on light perception.
It was hypothesized that Diadema specimens would take longer to travel into the shade when they had a sediment load, because their ability to sense light would be reduced, due to the obstruction of light-sensing cells on the test.
RESULTS
The time taken for the Diadema specimens to move into the shade without a sediment load was 1:45 minutes. When their test was covered with a layer of sediment, all urchins failed to move into the shade. Even after 10 minutes the urchins had not even begun to move from their start position. This could either suggest that the urchins were unable to sense the change in light intensity due to the obstruction of the light-sensing cells on the test, in which case the hypothesis was supported. Alternatively, the urchins may have been so stressed by the sudden increased sediment load that they did not exhibit typical shade-seeking behaviour.
DISCUSSION
Diadema have light-sensing cells on their test, spines, tube feet and pedicellariae (Rogers et al, 2004). By eliminating the ability of the pigment cells on the test of the urchin to sense light, the effect that increased sediment load may have on these urchins was assessed. Results suggested that Diadema with increased sediment load did not perceive light, and failed to seek shade, and thus the hypothesis was supported. However this result is unsupported by previous research on Diadema, which showed that even though the ambulacral margins were identified to be the most sensitive organ to light, the spines, pedicellariae and tube feet also responded (Millot, 1954). The urchins in our experiment should have responded to light even when covered with sediment, because the spines and tube feet were still exposed. This suggests that an alternative explanation of results is needed.
An alternative interpretation of the results could suggest that the urchins were highly stressed by the sediment load and failed to exhibit normal shade-seeking behaviour. If this were the case, the hypothesis that Diadema would respond less to light when covered in sediment was supported, but not for the reasons expected. Regardless, it is apparent that increased sediment load may have a detrimental effect on the ability of Diadema to seek shelter, either by reducing their ability to perceive light, or by increasing stress to such high levels that behaviour changes.This study is limited however by its small sample size, so all conclusions drawn should be considered in context and not over-extrapolated.
If Diadema are unable to find shelter, they are exposed to predation and excessive wind action, which may lead to increased mortality and a decline in urchin numbers on the reef. This would have a cascade effect on the reef ecosystem. Decreased urchin abundance would lead to a decrease in herbivory on the reef, and an increase in algae. An increase in algal biomass may cause a phase shift in dominance, leading to an algal-dominated reef. Corals are thus outcompeted by algae, and the loss in calcium carbonate due to erosion is not replaced rapidly enough by calcifying corals or algae, and the reef begins to degrade. The health of the reef is therefore reliant on the presence of herbivores such as Diadema.
In conclusion, this study illustrated that the dredging of Gladstone Harbour and the resulting sediment load could have detrimental effects on the adjacent reefs. Further study could be performed on a large scale to determine the effect of increased sediment load on other reef organisms.
|